Indian Scientists have developed a method to produce hydrogen with high purity (99.99%) from methanol-water mixture at ambient pressure and temperature that uses only one-third of the electrical energy required in water electrolysis.
With near-zero or zero end-use emissions and continually replenished fossil fuel resources, hydrogen can be an ideal sustainable energy carrier and would play an immense role in the near future. Hydrogen is gaining much attention due to its high specific energy value of 40 kWh/kg as compared to chemical fuels like gasoline, diesel, liquid petroleum gas (12-14 kWh/kg). The most abundant raw material containing hydrogen is water. It is also present in natural gas, petroleum, and biomass, and they can form the source for generation of hydrogen.
Water electrolysis and reformation of hydrocarbon like methane are common methods for production of hydrogen. For India’s energy transition to clean fuels, adoption of green hydrogen from renewable energy, integrated water electrolysis process to generate energy would bring in significant benefits.
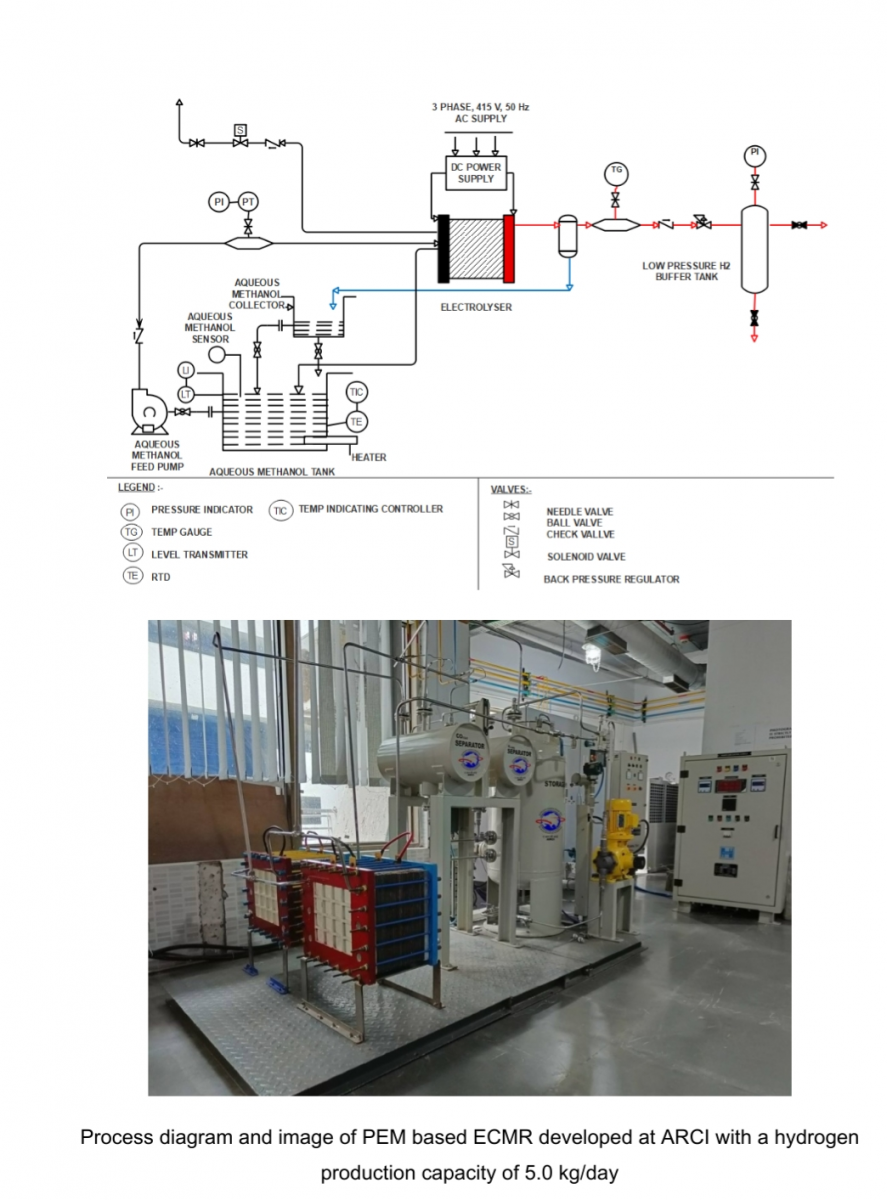
Scientists at the International Advanced Research Centre for Powder Metallurgy & New Materials (ARCI), an autonomous institute of the Department of Science & Technology, Govt. of India, have developed a method which combines both the processes of electrolysis and reformation to produce hydrogen from methanol-water mixture by electrochemical methanol reformation (ECMR) at ambient pressure and temperature. The main advantage of this process is that the electrical energy needed to produce hydrogen is 1/3rd of water electrolysis (Practical water electrolysis requires 55-65 kWh/kg of hydrogen). This technology has been patented by ARCI (Indian Patent 338862/2020 and 369206/2021).
In the ECMR process, which uses polymer electrolyte membrane (PEM), hydrogen can be produced at a lower temperature (25-60oC) and pressure, unlike chemical reformation. Hydrogen separation or purification steps are not required since it is being well separated from CO2 by polymer membrane used in the system. ARCI team is working on this technology and have developed an electrolysis unit of upto 5.0 kg/day capacity to produce hydrogen. The corresponding energy requirement for the electrolyser stack is around 17 kWhr/kg. The hydrogen thus produced by ARCI is highly pure (99.99%) and can be directly used in PEM fuel cells to generate power of about 11-13 kW.
The core components of the PEM-based ECMR electrolyser stack were fabricated indigenously and integrated with other components in the system. The electrolyser stack was fabricated using exfoliated graphite material as reactant flow field plate. Use of carbon materials as bipolar plates has been one of the significant achievements in replacing the titanium plates, which is otherwise normally used in electrolyser unit assembly, offering a conservative cost-benefit.
ARCI team has developed the indigenous process for fabricating the core components like Membrane Electrode Assembly (MEA), bipolar plates, and several process equipment. This method will significantly reduce the hydrogen cost compared to the water electrolysis method and can be easily integrated with renewable energy sources. ARCI is working with industry partners for integration with renewable energy sources like PV.
Patent details : (Indian Patent 338862/2020 and 369206/2021).
For more details contact Dr. R. Balaji Senior Scientist, Centre for Fuel Cell Technology, ARCI [rbalaji(at)arci(dot)res(dot)in].