Proteins, which are assemblies of numerous atoms forming the building blocks of life, demonstrate cognitive-like responsiveness.
Traditionally, intelligence is thought of as a trait exclusive to complex higher organisms with nervous systems like humans and apes. It involves cognition, learning, and the ability to respond to stimuli with adaptation or intention.
A team from Bose Institute, an autonomous institute of the Department of Science and Technology (DST) set out to examine whether a molecule composed on an assembly of atoms mimic intelligent behaviour at the very basic level.
The research led by Prof. Shubhra Ghosh Dastidar, involving his student Nibedita Ray Chaudhuri, worked on TAK1 kinase, a protein known for its role in cellular stress signaling and crucial to immune response, inflammations and even for the survival of cells. They observed that such highly organized assembly of atoms in specific cases, can develop such potential, even at a very rudimentary level.
The discovery, which is now published in Journal of Chemical Information and Modeling is an outcome of the handshaking between Biochemical research and Machine Learning (ML); the latter is a subset of Artificial Intelligence (AI) based methods. The work is a classic example of an interdisciplinary approach and forms a part of a trilogy on TAK1, published during 2023-2025.
Proteins are composed of thousands to millions of atoms suitably bonded to form polymeric chains of compounds called amino acids, but they become functionally alive only when those chains fold into a specific 3D compact form, called native state. It happens through the formation of enormous number of tiny electrostatic interactions between the atoms.
The collective pattern of this internal wiring is unique to a specific protein whose memory is coded into its so called ‘primary sequence’, and it gets updated across evolution yielding newer characteristics.
The findings of the researchers of Bose Institute highlight that this internal wiring in TAK1 not only makes it ‘functional’ but also creates a pseudo-intelligence enabling it to deploy its own machinery in a context dependent manner.
The internal wiring of interactions can process signals like chemical modifications as well as remotely sensed physical inductions by another molecules through different routes of its internal circuits of interactions, to trigger the TAK1’s function.
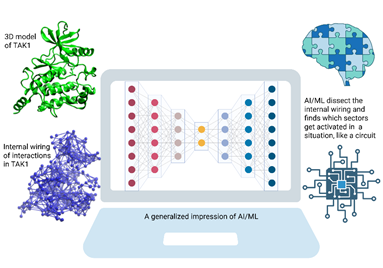
Fig : A schematic representation, showing the TAK1 structure as well as its internal atom-atom interactions. AI/ML, working in a computer, looks at it to find how the internal wring responds in a context dependent manner. [Created in BioRender. Ghosh Dastidar, S. (2025) https://BioRender.com/y0zf9lh]
TAK1 kinase is extremely important for immune response, inflammations and even for the survival of cells, and so its machinery is a target for drug molecules.
While the identification of the intelligent machinery of an important drug target itself opens up enormous possibilities of its future applications towards human benefit, it is also of fundamental importance in the biochemical science to extend the dogma of ‘sequence-structure-function’ to the possibility of ‘sequence-structure-function-intelligence’ for molecule specific cases.
Publication link: https://doi.org/10.1021/acs.jcim.5c00529
For more details, contact Prof. Shubhra Ghosh Dastidar (Email: sgd[at]jcbose[dot]ac[dot]in).






























