Hydration dynamics of proteins plays a pivotal role in the aggregation of several proteins which is a preliminary step towards various neuro-degenerative diseases. Thus aggregation process could be spotted early by detecting altering water network dynamics and modulated using inactive substances that serve as the vehicle or medium for a drug or other active substance.
Understanding debilitating neuro-degenerative diseases at the molecular level is crucial to find treatments or solutions for them. A phenomenon called ‘liquid liquid phase separation’ (LLPS) underlines the formation of cells organelles like P bodies, nucleolus which are membrane-less compartments in the cytoplasm of cells. LLPS, a self-aggregated system, is an intermediate step during the formation of the stable protein aggregates. When multivalent proteins interact they undergo rapid transformation from small complexes to large polymeric assemblies with increase in protein concentration. This dense phase often resembles liquid droplets exhibiting higher protein density and weaker molecular motion than the surrounding medium. This process, initiated through liquid liquid phase transfer, plays crucial role in inducing human diseases, especially age-related neuro-degenerative diseases like Alzheimer’s disease, Parkinson’s disease and cataract. Therefore, understanding the process of phase separation at a molecular level has become an emergent area of research in molecular biology fraternity.
Scientists at the S.N Bose National Centre for Basic Sciences, an autonomous institute of the Department of Science and Technology (DST) have explored how the hydration of proteins, gets altered as LLPS sets in. The researchers have spotted the crucial role of water in Liquid liquid phase separation which holds the key to neuro-degenerative diseases. They found that some excipients or inactive substance that serves as the vehicle or medium for a drug or other active substance like sucrose can stabilise LLPS while some can inhibit it. Thus aggregation process of these diseases could be modulated by altering water network dynamics using these excipients.
In a paper published in J. Phys. Chem. Lett the scientists, under the leadership of Prof. Rajib Kumar Mitra, examined four excipients ---arginine, glucose, ubiquitin, and bovine serum albumin. Some excipients like sucrose were found to stabilize the LLPS process while Bovine Serum Albumen (BSA) was found to inhibit the process.
Their experiments have identified that both protein and excipient hydration are important in regulating the LLPS process. Monitoring a change in hydration could therefore act as a potential marker for an early and easy detection of LLPS onset.
Publication link: https://doi.org/10.1021/acs.jpclett.1c03449
For more details, contact Prof. Rajib Kumar Mitra, Email : rajib[at]bose[dot]res[dot]in .
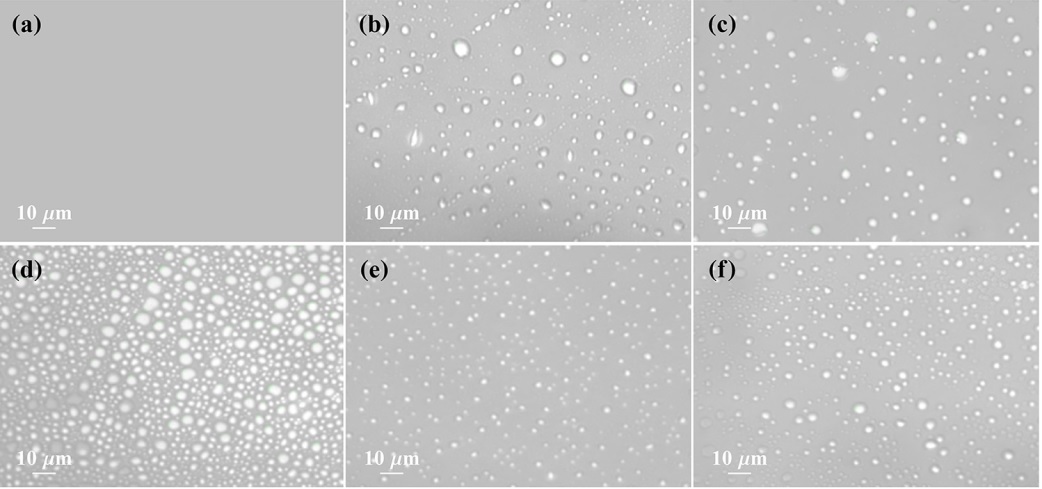
Figure 1:Microscopic image of Lys under different conditions: (a) No phase separation, (b) only Lys (600 μM), (c) Lys−100 mM Argsolution, (d) Lys−10 mM sucrose solution, (e) Lys−50 μM BSA solution, and (f) Lys−100 μM Ubi solution. All of the measurements were carriedout after 6 h of incubation at 50 °C. The concentration of Lys was kept fixed at 600 μM, and the pH of the solution was 12.6.
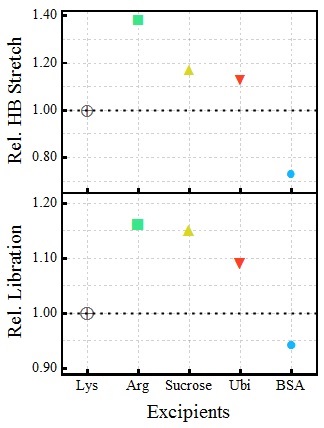
Figure 2. Relative change in the overall hydration during the LLPS formation of Lysozyme in presence of excipients (considering LLPS in Lys as the unity for both HB stretching and librational mode)