A change in the shape or structure of certain catalysts can improve their efficiency in catalyzing reactions, according to a new study.
Catalysts are essential in many chemical, biological, and industrial processes as they can enhance reaction rates, improve efficiency, provide selectivity, promote sustainability, offer versatility, and have biological significance. They are indispensable tools and have a significant impact on our life. In general, metal nanocomposites are efficient catalysts with numerous advantages, including high surface area, tunable properties, enhanced stability, versatility, green and sustainable catalysts, and catalyst cycling.
Scientists from Institute of Advanced Study in Science and Technology (IASST), Guwahati, an autonomous institute of the Department of Science and Technology (DST), led by Prof. Devasish Chowdhury, initiated a study to understand, by experiment and through a simulation method called Finite Difference Time Domain (FDTD) simulation, how to improve the catalytic efficiency of the catalyst. Nur Jalal Mandal, JRF, and Rahul Sonkar, JRF, are the group members involved in this study. In this work, a comparative study was done to investigate the catalytic reactivities of gold nanorod-molybdenum disulfide quantum dots (AuNR-MoS2 QDs) composite, which has a rod-like structure and gold nanosphere-molybdenum disulfide quantum dots (AuNS-MoS2 QDs) composite. The catalytic efficiency of the as-prepared composites was then investigated to reduce p-nitrophenol, taken as the model reaction.
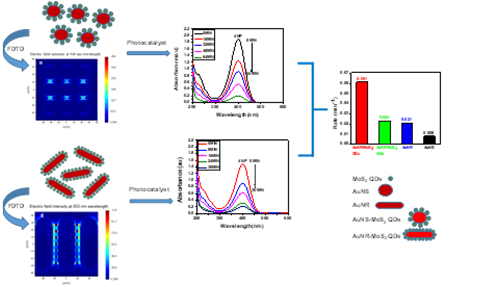
The kinetics of the catalytic reduction of p-nitrophenol reveals that the AuNR-MoS2 QDs composite demonstrates the highest catalytic efficiency, emphasising the role of the structural aspect. In addition, Finite Difference Time Domain (FDTD) simulation indicates that, in general, the electric field intensity increases for gold nanorod-molybdenum disulfide quantum dots composites when compared with only gold nanosphere-molybdenum disulfide quantum dots composite, gold nanorod, and gold nanosphere. “Such study will help scientists design more efficient catalysts, especially for photodegradation of dyes or polyaromatic hydrocarbons,” says Prof Chowdhury.
The finding was recently published in ACS Applied Nanomaterials https://doi.org/10.1021/acsanm.3c00475
For more details contact Prof. Devasish Chowdhury (devasish[at]iasst[dot]gov[dot]in)






























