A newly synthesized crystalline, chemically stable material, belonging to the class of crystalline porous organic polymers with permanent porosity and highly ordered structures, could enhance energy density of found to be ideal as a cathode for Li-S batteries and make them more efficient.
Lithium-ion batteries are a crucial requirement for weaning off the centrality of fossil fuels. But there are challenges in the route to its widespread use. Firstly, lithium is a relatively rare metal. Secondly, they have a low energy density, which means that these batteries have a shorter run time. In most lithium-ion batteries the energy density ranges from 50 Wh/kg to 250 Wh/kg - insufficient for long-term applications. Scientists are therefore been looking for alternatives to lithium-ion batteries.
Under these circumstances, lithium-sulphur (Li-S) batteries, consisting of a lithium anode and a sulphur cathode are emerging as a low-cost alternative energy storage technology. Li-S batteries have an energy density of around 550 Wh/kg, which is very useful for practical applications. However, for widespread and varied use, some shortcomings, like low cycle life and poor power delivery need to be overcome.

Figure 1. Li-ion battery cells
Dr. Pradip Pachfule and his team at the Extended Crystalline Organic Materials (ECOM) Laboratory at S. N. Bose National Centre for Basic Sciences, Kolkata, are addressing these problems by experimenting with porous organic cathode materials as sulphur hosts. Working with a class of compounds called Covalent Organic Frameworks (COFs) they have synthesized a nitrogen-rich crystalline, chemically stable triazine-based crystalline porous organic polymer material with a highly ordered structure. The structure is made up of organic monomers linked together by strong covalent bonds to form ordered and uniform channels. The precise arrangement of the light atomic building blocks resulted in a framework structure with an extensive network of interconnected pores.
These pores provide a large surface area, making ample space for the storage and movement of ions within the COF material. This feature promotes faster charge and discharge rates, increasing battery performance, and enabling rapid energy delivery. The crystalline structure of the COF gives the battery greater stability compared to amorphous materials. In addition, the high tunability of the COF allows materials to be designed with tailored properties to meet application-specific requirements. As a result, COFs have proven to be a promising cathode material.
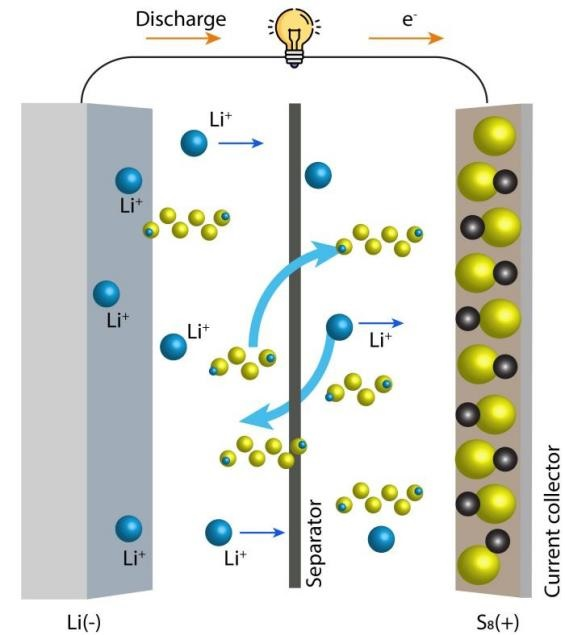
Figure 2. Working principle of lithium-sulfur battery
"Nitrogen-rich materials such as porous polymers, carbon nitrides, poly (triazine imide) and covalent triazine frameworks (CTFs) have been investigated as electrode materials for batteries and have often shown high energy densities. It is expected that the synthesis of a crystalline, porous and chemically stable triazine-based COF with additional redox-active moieties would greatly enhance their energy density as a combined effect of the presence of high-density redox-active sites in the conjugated and robust network," Dr Pachfule's team observe in their research published in the journal Angewandte Chemie International Edition.
The team prepared a COF in the 1:1 mixture of a polar and a non-polar solvent at 120°C for 3 days by reacting 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline (TTT) and 4,8-dimethoxybenzo[1,2-b:4,5-b′]dithiophene-2,6-dicarbaldehyde (DMTD) in the presence of an acid catalyst in a sealed tube. After cooling to room temperature, a red-colored precipitate was collected by filtration, washed with methanol and acetone and finally dried under atmospheric conditions. The imine (a functional group or chemical compound containing a carbon-nitrogen double bond) COF was then converted to thiazole-linked (a heterocycle containing sulphur and nitrogen atoms) COF in a one-step reaction. The resulting material was found to be ideal as a cathode for Li-S batteries. The increased porosity of the material provided a high surface area for reaction and sulphur loading, and functional groups such as triazine to react well with lithium - helping to transfer Li ions more efficiently than the neutral carbon in the previous S-C cathodes. The energy storage capacity and durability of these batteries were tested. The COF-based organic batteries showed a high capacity of 642 mAh/g at 1.0 C and durability, maintaining 78.9% capacity retention after 200 cycles. Tests are ongoing to further improve the performance.
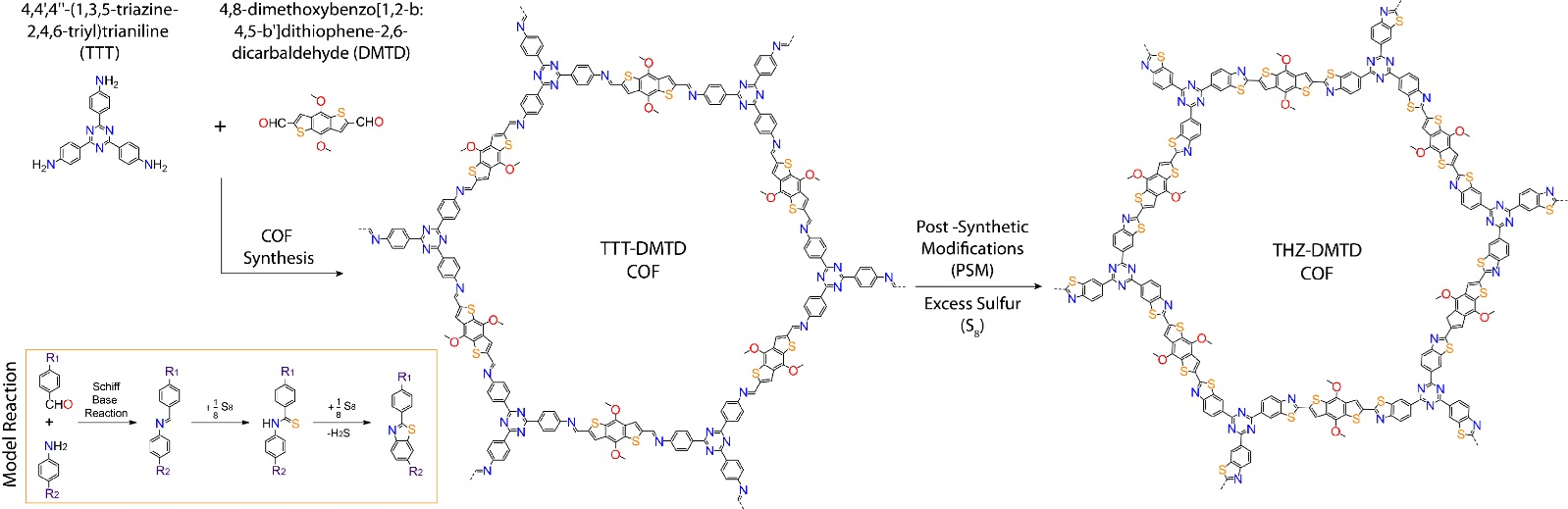
Figure 3. Synthesis of crystalline and porous covalent organic frameworks
After testing different permutations and combinations of the building blocks of COFs to see which combination gives the best results for Li-S batteries, the team produced an efficient and robust organic material suitable for the cathode. The next step would be to synthesize the COF with the required properties on a large scale for efficient, durable, affordable and stable Li-S batteries of green batteries that are the key to our sustainable future.
Publication link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202302276
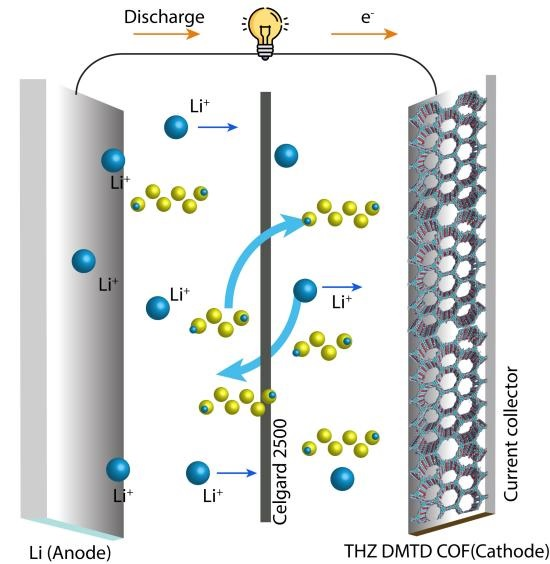
Figure 4. Working principle of lithium-sulfur battery with COF cathodes






























