A newly designed catalyst consisting of an alloy of cobalt, manganese, and tin is more stable and can produce hydrogen through electrolysis of water more efficienly than the individual metals or binary (Co-Mn, Mn-Sn or Co-Sn) alloys.
Water electrolysis is a method of producing hydrogen by splitting water molecules into hydrogen and oxygen using electricity. The process offers a clean and sustainable approach to produce hydrogen, which is essential for various industries and clean energy technologies.
The efficiency of this process relies on catalysts that accelerate the hydrogen evolution reaction at the cathode. Currently, platinum-based catalysts are widely used but come with drawbacks such as high cost, scarcity and slow reaction rates, limiting their widespread adoption for water electrolysis.
Scientists from the Centre for Nano and Soft Matter Sciences (CeNS), Bangalore, and the National Chemical Laboratory (CSIR-NCL), Pune, have developed a promising catalyst for the hydrogen evolution reaction (HER), a crucial step in water electrolysis for producing hydrogen. This newly designed catalyst, a mixture of cobalt, manganese, and tin known as Co-Mn-Sn alloy, has shown better efficiency and stability in generating hydrogen as compared to the individual metals or binary (Co-Mn, Mn-Sn or Co-Sn) alloys. The presence of manganese and tin in the alloy played a synergistic role in boosting its performance. As the Co-Mn-Sn alloy does not contain any platinum group metal it offers exciting prospects. This work was recently published in Elsevier- International Journal of Hydrogen Energy.
This study is part of an ongoing project to develop multicomponent alloy catalysts which is supported by the Science and Engineering Research Board (SERB), an attached institution of the Department of Science and Technology.
This innovation brings us a step closer to efficient and eco-friendly hydrogen production through water electrolysis. With the Co-Mn-Sn alloy catalyst, the obstacles to using renewable energy for large-scale hydrogen generation could be significantly reduced. As the demand for clean energy continues to rise, this advancement could pave the way for a greener and more sustainable future.
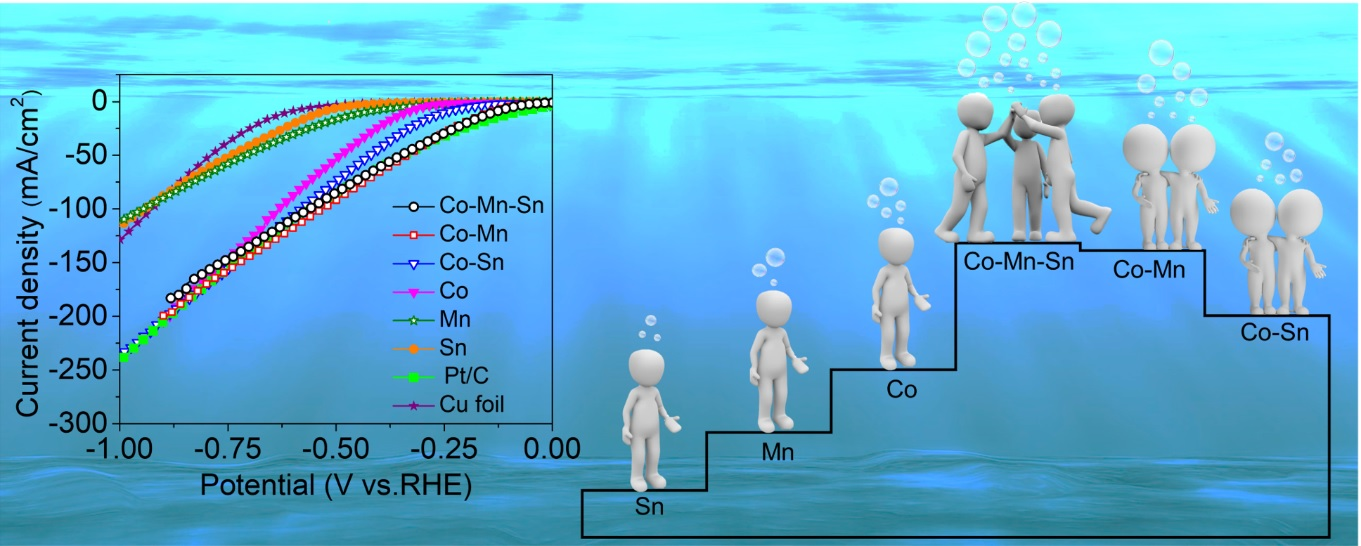
Figure: Linear Sweep Voltammetry polarization curves of hydrogen evolution reaction (HER) in alkaline electrolyte for various catalysts and their comparison with CoMnSn alloy; schematic representation shows the advantage of multicomponent alloys to achieve favourable entropy for HER.
Publication details: DOI: https://doi.org/10.1016/j.ijhydene.2023.07.064
For further details, Dr. Ashuthosh Kumar Singh ( Email: aksingh[at]cens[dot]res[dot]in) or Prof. B L V Prasad (Email: pl.bhagavatula[at]cens[dot]res[dot]in) can be contacted.






























