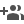A new model for generating mass of neurovascular tissues or neurovascular organoids/embryoids (NVOEs) from autologous blood can help in the investigation of impaired brain functioning and development by analysing in neuroimaging (preclinical) scans, correlating with altered blood supply.
The field of neural organoids is rapidly progressing and has fueled the hope (and hype) for improved understanding of brain development and functions, modeling of neural diseases, discovery of new drugs, and supply of surrogate sources of transplantation. Most such neuronal organoids are derived from genetic overexpression of embryonic/extraembryonic transcription factors, and they lack vascularization. As an advancement a new approach of co-culturing blood vessel organoids with cerebral organoids was recently proposed, they lacked active blood flow and are very laborious and not cost-effective. The most advanced embryo models with neurogenesis /organogenesis also lack functional vasculature and hence have limited scope for modeling brain-activity based investigative studies.
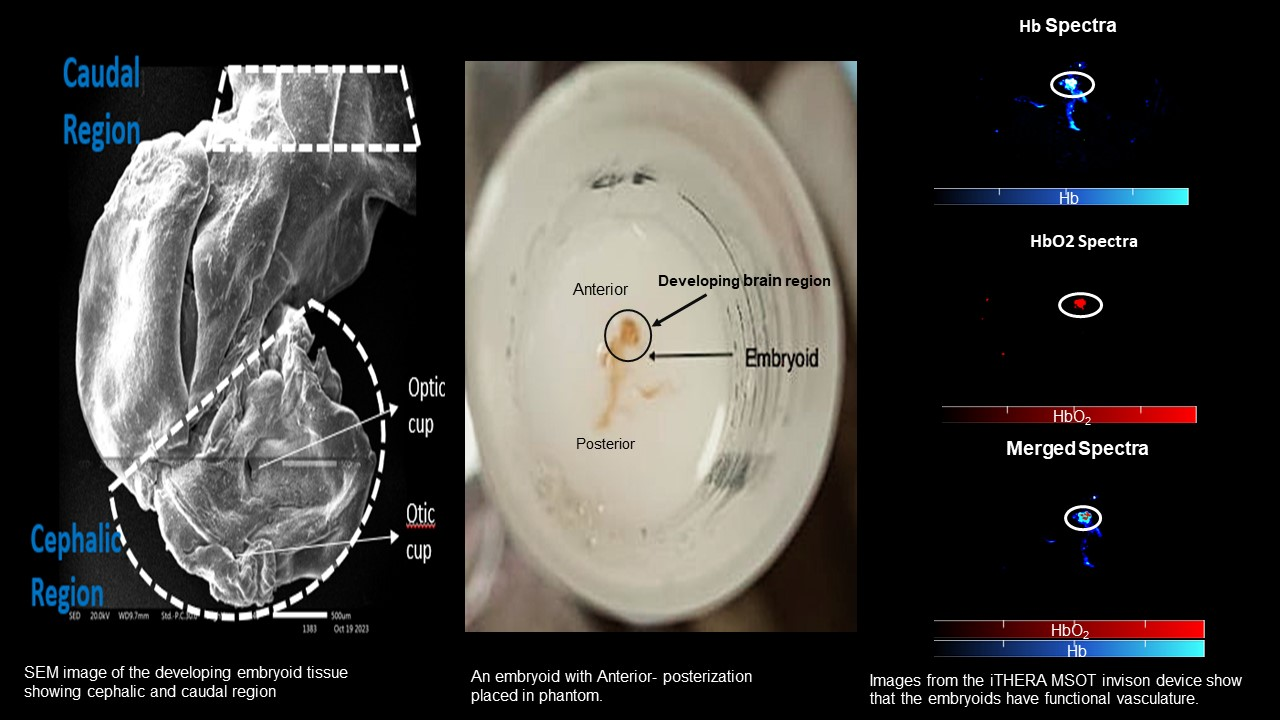
As an answer to this challenge, researchers of Post Graduate Institute of Medical Education & Research, PGIMER, Chandigarh have come up with a prototype for establishment and characterization of novel self-organizing neurovascular organoids/ embryoids (NVOEs) entirely from autologous blood without any genetic maneuvering or morphogen supplementation.
The research funded by Anusandhan National Research Foundation (ANRF-erstwhile SERB) can produce functional vascularised embryoids on its own and requires no guided patterning. It is cost-efficient as it requires no specific differential media, growth factor or differentiating morphogens for culturing, but only autologous plasma and blood cells.
The existence of a functioning vasculature in neurovascular embryoid was verified by detecting both haemoglobin (Hb) and deoxy haemoglobin (HbO2) signals using the BOLD (Blood-Oxygen-Level-Dependent) signal concept. (data attached).
The implications are vast for studying neurological (neurosensory/motor and neuro-immune) disease pathways, neuroregeneration, preclinical neuroimaging, and endogenous gene editing, and autologous immunotherapies for tumors, and autoimmune diseases.
The researchers who are in the process of filing a patent for this at the Punjab State Council for Science and Technology, Chandigarh, are employing these models to understand the genetic basis of neurosensory hearing loss and auditory comprehension challenges (altered central auditory activity) in children with congenital Sensorineural Hearing Loss (SNHL) (early onset), with and without comorbid features (autistic- like behaviour) or neurodevelopmental defects (intellectual disability, ANSD, language disorders). These children have poor Cochlear Implant communication outcomes that encouraged the team to investigate the altered higher/central auditory activity pathways by generating embryoids marked by neurovascular coupling; the fundamental phenomenon that drives sensory (peripheral)-evoked brain (central) activity. Functional neuroimaging tools like fMRI is only clinical tool that can be employed to monitor altered brain activity at our health care centre, however, it is currently not actively used for congenital SNHL, ANSD or autistic children due to incompatible magnet-based cochlear implants or hyperactivity of kids that dissuades fMRI owing to motional artifacts or requirement for prolonged anaesthesia in these kids.
The prototype can help develop patient-specific embryoid models (precision medicine) for congenital neurosensory, neurodevelopmental and neurodegenerative diseases like Autism, ADHD, ANSD, Alzheimer’s and Parkinson's. It can also be used for deciphering genetics and neural circuits, testing drugs bypassing the blood-brain barrier, and identifying novel biomarkers for early neurological diseases.
The birth of neurons: Autologous blood generated neurovascular embryoids with implications in precision medicine treatment