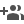Researchers have made a rare observation where the local crystal structure symmetry or the arrangement of atoms in the immediate vicinity of a given atom, in a crystal, reduces upon warming, contrary to the usual trend of symmetry of crystal structures increasing with rising temperatures. The study underlines the significance of chemical design in triggering unconventional phenomena in crystalline materials useful for phononics, thermoelectrics and solar thermal conversion.
Symmetry breaking plays a crucial role in fundamental chemistry and physics. A familiar manifestation of this phenomenon is the transition of a gas to a liquid and eventually to a solid upon cooling, with each phase transition involving a reduction in symmetry.
Thermodynamic factors like entropy (measure of disorder) and enthalpy (measure of total energy stored) of a system determines how the system responds to changing conditions like temperature fluctuations.
Traditionally, it is believed that as a material is heated, it tends to adopt a higher crystal symmetry due to the favorable increase in entropy.
However, recent findings by Prof. Kanishka Biswas, Ms. Ivy Maria, Dr. Paribesh Acharyya and other team members at Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bangalore, an autonomous institute of Department of Science and Technology, challenge this conventional understanding, especially at the local structural level of a crystal.
Local structure of a crystal is the arrangement of atoms in the immediate vicinity of a given atom in a crystal, typically within the collection of the first and second nearest neighbour atoms around a specific atom, technically known as the first and second atomic coordination environments respectively.
In an ideal crystal, the local structure mirrors the global structure, but in certain rare cases, they can diverge. This is precisely what the team observed in an all-inorganic two-dimensional halide perovskite, Cs2PbI2Cl2 that belongs to the family of Ruddlesden-Popper halide perovskites (class of materials with a specific crystal structure).
Contrary to the usual trend where heating increases symmetry, this compound exhibits a decrease in local symmetry with rising temperature, while the global crystal symmetry remains unchanged. This occurs due to configurational averaging, where the distorted local symmetries average out at longer length scales, leaving the global structure intact.
This phenomenon of local symmetry breaking upon heating is termed "emphanisis," meaning "appearing out of nothing." The team employed an advanced synchrotron X-ray technique which simultaneously reveals both the local and global structures of solids from their X-ray diffraction patterns, to investigate emphanisis.
The synchrotron X-ray experiments were done in DESY, Hamburg, Germany under the India-DESY collaboration supported by Department of Science & Technology (DST), India.
The researchers traced this unusual local symmetry breaking to the stereochemically active lone pair of lead in the compound.
Interestingly, Cs2PbI2Cl2 accommodates two types of structural distortion -- static distortions in chlorine atoms and dynamic distortions in lead atoms. These distortions result from the complex interplay between different structure-distorting effects driven by the interactions between the mixed halide (Cl and I) motif and the active lone electron pair of lead in Cs2PbI2Cl2. The distortions happen because of a competition between a mix of structure-distorting forces that arise because of interaction of different parts of the material (the mixed anions- Cl and I) with the lone electron pair on the lead atoms in Cs₂PbI₂Cl₂.
The high temperature "emphanitic" phase is characterized as a disordered distorted state, existing at the intersection of an ordered undistorted state and an ordered distorted state.
"Emphanisis" is a promising strategy for achieving intrinsically low lattice thermal conductivity in crystalline materials. Such materials are highly sought after for their fundamental importance and diverse applications, including phononics, thermoelectrics, solar thermal conversion, and various heat management systems.
The study now published in Advanced Materials, underscores the fundamental and functional significance of chemical design in creating unconventional phenomena in crystalline materials. The findings suggest that understanding these thermodynamic subtleties can lead to intriguing structural transformations with broad applications.
Publication link: https://doi.org/10.1002/adma.202408008
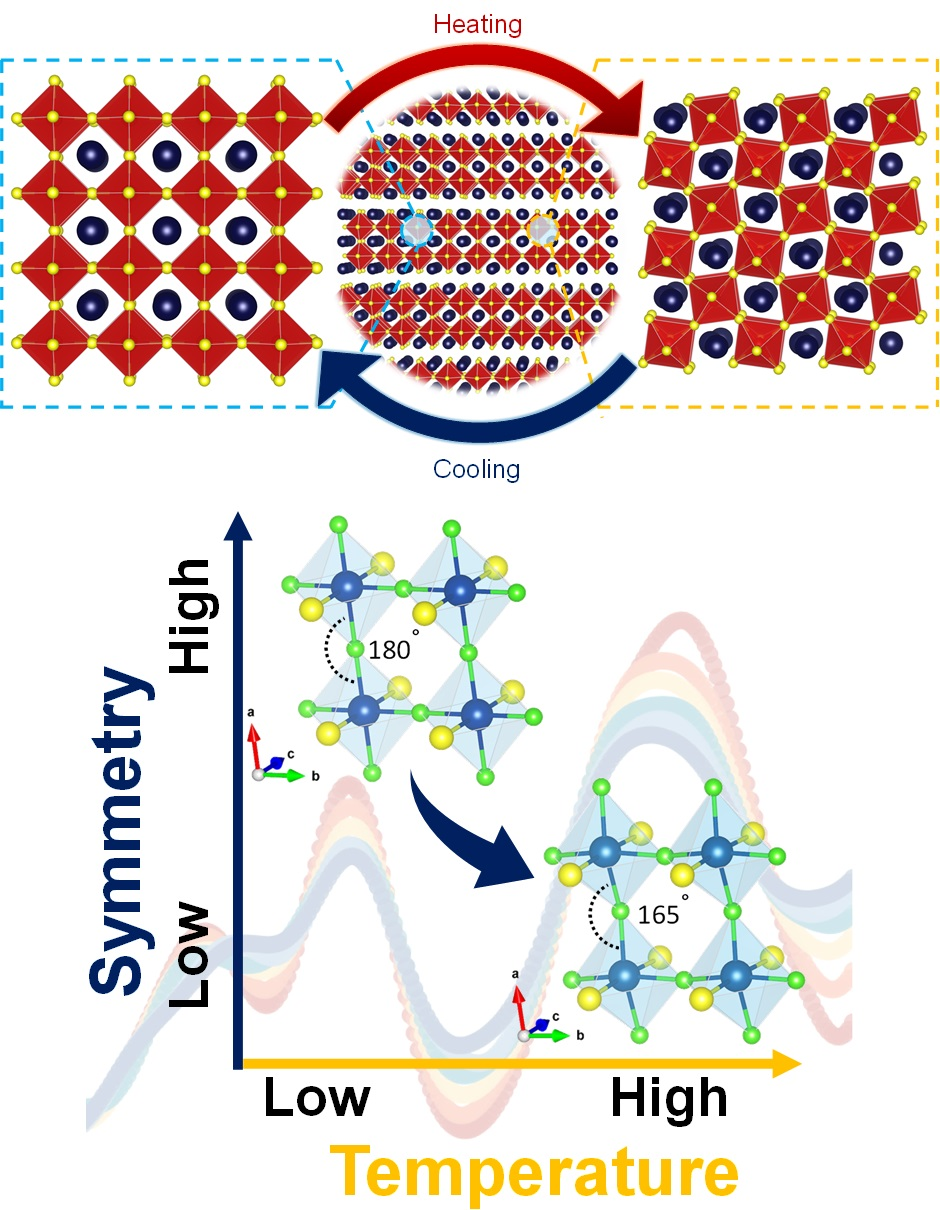
Figure 1. Schematic representations showing the evolution of local structure of a system exhibiting emphanisis.

Prof. Kanishka Biswas (left) and Ivy Maria (right) at Solid State Chemistry Lab, JNCASR, Bangalore