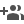Researchers have found a new way to utilise carbon dioxide CO2 in ambient reaction conditions, unlike formerly reported harsh thermal conditions. Using CO2 they have converted amines to N-formamides useful for synthesizing heterocycles, pharmaceuticals, and bio-active compounds, through a green approach.
Carbon capture and utilisation is becoming more and more important as a solution for the rising green house gases in the atmosphere. Polyoxometalates (POM), a class of synthesised nanomaterials, consisting three or more transition metal linked together by shared oxygen atoms, are promising candidates for improving photocatalytic conversion of CO2 which is the most significant green house gas.
They offer high-efficiency catalytic sites and exhibit extraordinary thermal stability, redox ability, and semiconductor-like properties. Utilizing POMs as photocatalysts offer several advantages. Their light absorption properties can be finely tuned by incorporating different transition metals. The secret that makes them candidates for photocatalytic conversion are their properties of quick and reversible multielectron transfer. However, earlier most photocatalytic conversions have been carried out under extreme conditions and scientists are on the look out for solutions to carry out such conversions under normal conditions.
Researchers at Institute of Nano Science and Technology (INST) Mohali, an institute of Department of Science and Technology have explored two novel Keggin POM-based solids--- (C5H7N2)5[CoW12O40] (PS-96) and (C5H7N2)5[CuW12O40] (PS-97), [C5H7N2 = 4-aminopyridine] out of which the latter was found to be active for efficient and photocatalytic N-formylation of various substituted anilines and morpholine with CO2 using phenyl silane as a reducing agent, which operates under ambient conditions. They found that the band gap of the catalyst was ultra-low at 1.43 eV. This property specifically urged them to check the photocatalytic activity of POMs in the visible region.
Working in collaboration Dr. Suman Lata Jain, IIP Dehradun, INST researchers (Dr. Monika Singh, Parul Sood and others) found that in the N-formylation reaction, POMs can convert CO2 and amines into formamide derivatives by activating the CO2 molecule and promoting its reaction with the amine substrate. POMs exhibiting photocatalytic activity can initiate and accelerate chemical reactions under light irradiation. This property is particularly beneficial in the context of CO2 utilization.
Using POMs as photocatalysts aligns with green chemistry principles by reducing the need for stoichiometric reagents and minimizing waste while utilizing CO2 (a greenhouse gas) as a reactant. This contributes to more sustainable chemical processes. Moreover, POMs as photocatalysts are easily available and are cost-effective materials.
This research published in Journal of Material Chemistry A (https://doi.org/10.1039/D4TA02432J) opens the path for research investigating POM-based hybrid solids in photocatalytic N-formylation of amines using CO2.