 Indians will soon have access to the country’s first indigenous flow diverter stent for diverting blood flow away from localized ballooning of arteries in the brain and a device that promotes better healing of the hole in the heart.
Indians will soon have access to the country’s first indigenous flow diverter stent for diverting blood flow away from localized ballooning of arteries in the brain and a device that promotes better healing of the hole in the heart.
Nitinol-based occluder devices, which are presently used to heal Atrial Septal Defect (ASD) or hole in the heart that affects 8 out of every 1000 living babies born, are currently imported to meet demands.
Besides, currently, India does not manufacture flow diverters stents, which are needed for diverting blood flow away from an intracranial aneurysm or localized ballooning of arteries in the brain, helping reduce chances of its rupture and related stroke.
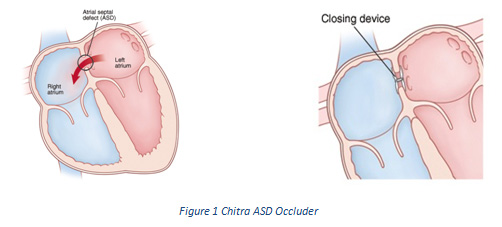 In order to address the challenges, Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), an autonomous institute of the Department of Science & Technology (DST), Govt. of India, under the Technical Research Centre (TRC), has entered into Technology Transfer Agreements with Pune based Biorad Medisys for two biomedical implant devices--- an Atrial Septal Defect Occluder and an Intracranial Flow Diverter Stents developed by the institute in collaboration with National Aerospace Laboratories, Bangalore (CSIR-NAL) using superelastic NiTiNOL alloys.
In order to address the challenges, Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), an autonomous institute of the Department of Science & Technology (DST), Govt. of India, under the Technical Research Centre (TRC), has entered into Technology Transfer Agreements with Pune based Biorad Medisys for two biomedical implant devices--- an Atrial Septal Defect Occluder and an Intracranial Flow Diverter Stents developed by the institute in collaboration with National Aerospace Laboratories, Bangalore (CSIR-NAL) using superelastic NiTiNOL alloys.
The Technology Transfer Agreements agreements were signed by Dr. K. Jayakumar, Director, SCTIMST, and Mr. Jitendra Hedge, Managing Director, Biorad Medisys, in the presence of Dr. Jitendra J Jadhav, Director, CSIR- NAL, through an online meeting on 14th Jan 2021.
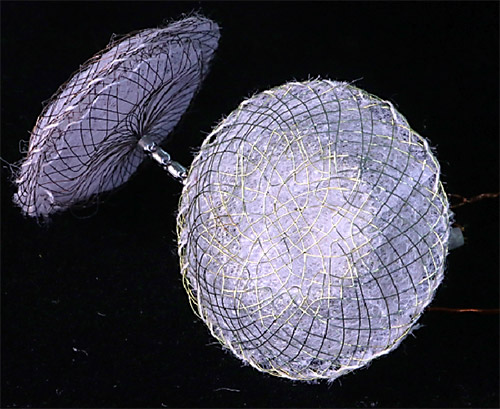 The novel ASD occlude developed by SCTIMST promotes better healing of the hole in the heart and also has softer edge for minimizing the damage to adjacent tissue. The delivery system has a novel release mechanism to enable smooth release of the device. The device is protected through two Indian patent applications, one international patent application, and design registration.
The novel ASD occlude developed by SCTIMST promotes better healing of the hole in the heart and also has softer edge for minimizing the damage to adjacent tissue. The delivery system has a novel release mechanism to enable smooth release of the device. The device is protected through two Indian patent applications, one international patent application, and design registration.
The flexible flow diverter stent that allows accurate positioning of the device across the aneurysm developed by SCTIMST is the first one to be manufactured in India. It possesses kink resistance and improved radial strength through a novel braiding pattern making the device flexible and adaptable to the distortion of the vessel boundaries. The device is also provided with radio-opaque markers for radiographic visibility. The associated delivery system allows accurate positioning of the device across the aneurysm. These features have been protected through two Indian patent applications, one international patent application, and design registration. The cost of the Chitra Flow Diverter stent is expected to be priced significantly lower than the currently imported ones.






























