Indian Scientists have developed a high-energy density aqueous supercapacitor with a wide electrochemical window, high stability as well as high energy retention.
With increasing focus worldwide to develop newer, highly efficient energy systems and promote renewable energy, there is growing attention on the role of supercapacitors and their applications in different sectors, including transportation.
Supercapacitors represent a unique class of energy storage systems and can play a critical role in the energy sector because of their inherent capacity for large number of charge and discharge cycles, high power density, rapid charging, and longer life span. However, they are often limited by lower energy density (amount of energy stored in a device), and scientists across the globe are looking to overcome this problem by developing new technologies and materials.
One of the ways to address the limitation of lower energy density is by fabricating metal oxide-based asymmetric devices wherein metal oxide provides higher energy density due to its electrochemically active behaviour. In addition, the use of aqueous electrolytes makes these devices safer due to their non-flammable and non-toxic nature.
Scientists at the International Advanced Research Centre for Powder Metallurgy and New Materials (ARCI), an autonomous R&D Centre of the Department of Science and Technology, Government of India, in collaboration with the Indian Institute of Technology, Hyderabad (IITH) have developed a supercapacitor system with 2.2 V wide electrochemical window and 83 % energy retention after 10,000 charge-discharge cycles. Scientists Samhita Pappu, Tata N. Rao, Surendra K. Martha, and Sarada V. Bulusu have chosen manganese oxide (MnO2) in this device. This stable electrode material is cost-effectiveness due to its abundance, environmental friendly, and electrochemically reversible.
Nanostructured MnO2 served as active, positive electrode material while the asymmetric supercapacitor device with commercial activated carbon as a negative electrode. The electrodeposited MnO2 exhibited an overall 2.2 V potential window. The charge storage, energy, and power density originating from MnO2 and carbon were further enhanced by the use of Potassium Iodide (KI), a redox additive in the electrolyte. With the optimized concentration of KI in the electrolyte, the asymmetric supercapacitor exhibited three times higher charge storage capability.
Any energy storage system is classified by its capacity to store charge (energy density) and dissipate charge (power density). In this regard, the designed 2.2 V aqueous supercapacitor could achieve a high energy density of 90 Wh kg-1 at a power density of 3300 W kg-1, with excellent capacity retention after 10000 cycles, indicating its promise for commercial applications. This research has been published recently in the journal ‘Energy’.
The device has been validated by successfully lighting 40 white LEDs and a DC fan for demonstrating its performance. The MnO2-based supercapacitor device is safe, cost-effective, and environmentally benign in comparison to commercial organic systems since it is an aqueous electrolyte system.
Further, the fabrication of pouch cells is underway for exploring its commercial viability.
Publication link:
Link: https://doi.org/10.1016/j.energy.2021.122751
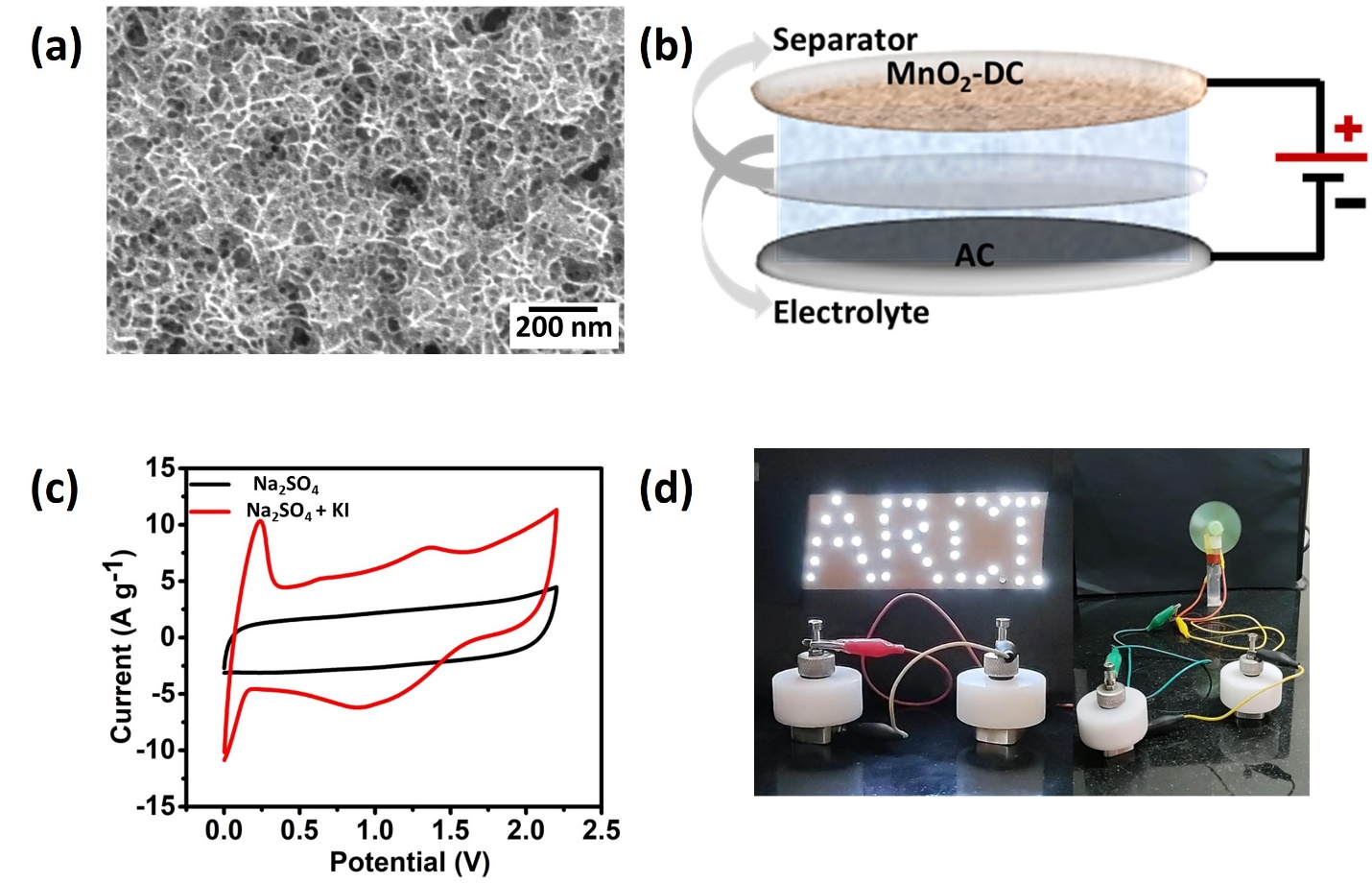
Figure 1: (a) Surface morphology of MnO2 nanosheets, (b) schematic of the design of 2.2 V aqueous asymmetric supercapacitor, (c) cyclic voltammetry profile with and without KI redox additive, and (d) LED, fan demonstration.






























