Scientists have synthesized a class of porous and crystalline organic materials called covalent organic frameworks (COFs) incorporated with quinone groups and sulphur-containing thiophene groups that can act as efficient and versatile electrodes for pseudocapacitors because of their low density, high stability, and well-defined atomic arrangements.
Supercapacitors are energy storage devices that can store more electrical energy than capacitors and transfer the stored energy faster than batteries. Although supercapacitors cannot store as much energy as batteries, they have qualities that make them a great asset in many devices. They can be charged and discharged an infinite number of times, giving them a virtually infinite life, and they charge and discharge much faster than batteries. They are also valued for their wide temperature range, environmental friendliness, improved safety, higher reliability and maintenance-free operation. Because of their unique properties, the search is on to make supercapacitors more reliable, more efficient and longer lasting by different methods like improving their electrodes. .
Currently, carbon-based materials such as graphene, which have excellent energy transfer properties, are commonly used as the electrode material for Thin Layer Chromatography (TLC). On the other hand, covalent organic frameworks (COFs) - a class of porous and crystalline organic materials have proven to be efficient and versatile electrodes for pseudocapacitors because these materials have very low density, high stability, and well-defined atomic arrangements.
Scientists from S. N. Bose National Centre for Basic Sciences (SNBNCBS), an autonomous institute of Department of Science and Technology (DST) have tailored their chemical properties by incorporating a wide range of organic functional groups into their backbone. The study published in Applied Energy Materials recently was led by Dr. Pradip Pachfule.
Dr Pachfule's team synthesized crystalline COF with dithiophenedione structures in its backbone (TTT-DHTD). The TTT-DHTD sample was prepared by a condensation reaction of two organic compounds -- 4,4¢,4¢¢-(1,3,5-triazine-2,4,6-triyl)trianiline (TTT) with 4,8-dioxo-4,8-dihydrobenzo[1,2-b:4,5-b¢]dithiophene-2,6-dicarbaldehyde (DHTD).
The redox-active quinones showed promise as a low cost, environmentally friendly, high energy density option for energy storage.
"We present a dithiophenedione-based COF that incorporates redox-active quinone groups and sulphur-containing thiophene moieties in a highly crystalline and porous COF. Due to these combined properties, the dithiophenedione-based COF exhibits excellent pseudocapacitive energy storage performance,” explains Dr Pachfule.
Evalution of the charge storage performance of the TTT-DHTD showed a stable, reversible, symmetric pattern– suggesting a stable supercapacitor performance. Further, the cycling performance of the TTT-DHTD COF displayed high capacitance after 2000 cycles, confirming the recyclability of COFs for supercapacitors. Also, the supercapacitor performance of TTT-DHTD was found to be superior compared to its other COF materials. The findings are a significant step forward in the production of high-quality supercapacitors.
Publication Link: https://pubs.acs.org/doi/10.1021/acsaem.3c01072?ref=PDF
Additional reference:
https://www.sciencedirect.com/topics/chemistry/supercapacitors
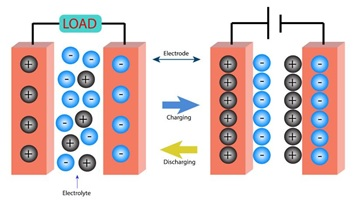
Figure 1. Schematic of a double-layer supercapacitor
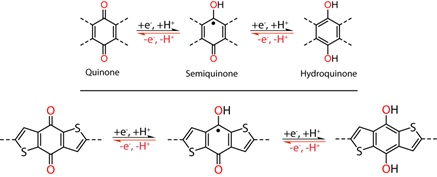
Figure 2. Electrochemical redox reactions of quinones