Indian Scientists have developed a highly efficient method for electrochemical synthesis of ammonia that may be useful for the industrial preparation of the chemical.
Traditional Haber-Bosch process of ammonia preparation utilizes huge energy in high temperature and high-pressure conditions for ammonia synthesis. Besides, the process also leads to emission of carbon dioxide, a greenhouse gas.
Electrochemical ammonia synthesis, on the other hand is absolutely ambient and can be carried out at room temperature and atmospheric pressure. Besides, it has a carbon-free approach and hence is a promising alternative to the famous century-old Haber−Bosch process. The major obstacle to this electrochemical process is the competitive hydrogen evolution reaction, occurring at the same voltage window as that of nitrogen reduction reaction (NRR), which results in a low yield and Faradaic efficiency (F.E.) for ammonia synthesis.
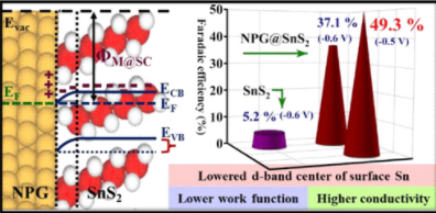
Scientists from INST led by Dr. Ramendra Sundar Dey have developed a new strategy called interface engineering by interfacing nanoporous gold (NPG) with Tin sulfide (SnS2) and extensively investigated the alteration in the electronic band structure of the hydrogen evolution reaction (HER) with high Faradaic efficiency of 49.3 % for ammonia synthesis. This is, till date highest among all the SnS2 based and interface engineering-based materials for NRR.
This work supported by DST SERB and DST INSPIRE funding agencies and published in the journal ‘ACS Nano’ is an attempt to upgrade the electronic properties of a semiconductor with a metal interface that not only help to suppress HER but also promote N2 adsorption at the active site and synthesize ammonia selectively with high Faradaic efficiency.
“Our research approach could pave path for the development of a catalyst, which would act well as an HER suppressant and is capable to compete with several high-performance catalysts to achieve a high Faradaic efficiency for NRR,” added Dr. Ramendra Sundar Dey.
The team explains, for any electrocatalytic process, the conductivity and d-band centre of the semiconductor could be improvised by interfacing the same with a metal.
The synthesis of NPG@SnS2 firstly involved cleaning of glass substrates successively with IPA, water, and acetone to do away with any kind of aerial impurity. This step was followed by the acid etching of the gold−silver alloy to obtain nanoporous gold (NPG), which was then transferred onto the glass substrates. This layer was coated with 100 nm thick Sn layer, which was then subjected to sulphurization in Ar atmosphere to obtain the semiconducting SnS2 layer over NPG and a metal−semiconductor interface at their junction.
INST team believes that the progress represented in this work is of immense importance to fine-tune the electronic properties of a semiconducting material with a suitable metal-semiconductor heterojunction according to the demands of the electrocatalytic process. The interfacial engineering strategy of catalyst development adopted in this work could be potentially used to achieve high Faradaic efficiency for electrochemical nitrogen reduction reaction by suppressing its competitive counterpart, that is, hydrogen evolution reaction (HER).
The scientists are yet try for industrial-scale ammonia synthesis with this material. But if the Faradaic efficiency for ammonia synthesis is considered, then this material can be surely worked upon for industrial preparation of ammonia.
Publication link: doi.org/10.1021/acsnano.1c08652
For more details, Dr. Ramendra Sundar Dey (rsdey[at]inst[dot]ac[dot]in) can be contacted.






























