The molecular mechanism behind the effectiveness of N-Acetyl-L-cysteine (NAC) and vitamin C, a medical combination used by doctors to treat kidney ailments, has been cracked in research conducted by a team of scientists. This study will help clinically treat chronic kidney ailments more effectively and help pharmaceutical research to develop better medicines.
High level of creatinine (CRN) in the blood stream is an indication of malfunctioning of kidneys. If the excess creatinine is allowed to remain in the blood, it may further damage the kidneys. This toxic chemical is not very soluble in water and thus it does not naturally get flushed out of the system. However, doctors have found that administration of doses of N-Acetyl-L-cysteine (NAC) and ascorbic acid (AS) or Vitamin C, help in reducing creatinine level in the blood stream. Doctors prescribe these antioxidants to many of those who are diagnosed with kidney ailment. However, the exact molecular mechanism of how NAC and ASC make CRN water soluble and thus excreted through urinary track was so far not clear.
A team from S. N. Bose National Centre for Basic Sciences, an autonomous institute of Department of Science and Technology, has undertaken the study of the interaction of NAC and ASC with CRN in an aqueous environment to understand how the NAC-CRN or ASC-CRN conjugate becomes water soluble.
The research led by Prof. Rajib Mitra found that CRN, in the presence of antioxidants, becomes strongly solvated compared to bare CRN. The study traced that the collective relaxation dynamics of water became much slower in the presence of the conjugate molecules implying stronger solvation present in the conjugates.
The investigation was carried out using terahertz (THz) spectroscopy, a technique in which the properties of matter are probed using transient (sub-ps) terahertz pulses, generated by focusing IR laser (less than 100 femtosecond pulse duration) incident on dipole antenna producing output THz radiation in 0.1-2 THz frequency region. The technique which provides information on both the amplitude and the phase of the radiation after passing through the sample has gained popularity in recent times in fields of biomedicine, food and agricultural products and security inspection.
Using this method, the research team has probed collective fluctuations of water dipoles in the pico-second to sub pico-second range and has gathered precise information regarding the solute (CRN-anti oxidant) hydration process.
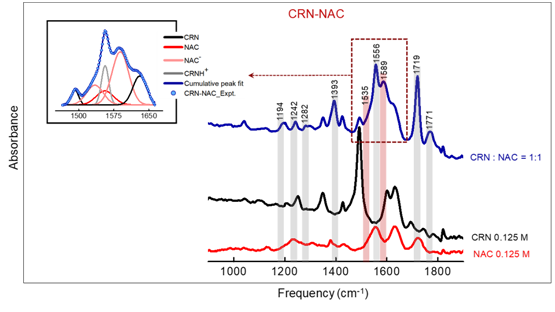
Fig 1. ATR-FTIR spectra of creatinine (CRN)-N-acetylcysteine (NAC) mixture in the mid-IR region along with the corresponding monomers with same effective concentrations. The newly found features with the corresponding peak positions are presented with light grey and red shades for CRNH+ and NAC-, respectively.
This investigation further render supports towards the proton transfer process as well as formation of ionized species within the medium. Such ionized molecule is soluble in water and hence could easily be flushed out of the blood stream through urine.
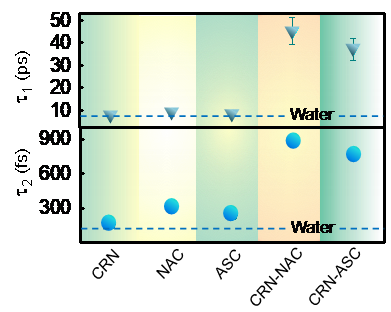
Fig 2: Relaxation time scales (‘Wait’ ( ) and ‘Switch’ ( )) for all the monomers and complexes and that of pure water has been shown with dotted line obtained from terahertz time-domain spectroscopic measurement.
The research which compared the H-bond stretching with pure water and solvation of bare monomers (CRN, NAC, ASC), concluded that the intermolecular bond is much stronger after the conjugate formation clearly suggesting the formation of strongly solvated conjugates.
This study asserts that now that the mechanism of the molecular level interaction is understood, systematic investigations (both experimental and simulation) can be run to search for better drugs which in turn will make treatment of renal problems more efficient.
Publication Link: https://pubs.acs.org/doi/10.1021/acs.jpcb.3c05334






























