Researchers are expanding the horizons of artificial enzymes known as “nanozymes” to use them as catalysts for transforming biomaterials for their futuristic use in medicinal and biomedical applications.
Several complex natural enzymes can act on proteins to generate functional proteins. However, the interplay of nanozymes with proteins has rarely been explored.
Scientists are now probing the unexplored roles of nanozymes in biological environments and their interplay beyond small molecule substrates due to their potential prospects in biotechnological and therapeutic interventions. They are also trying to develop next-generation artificial enzymes to overcome the current limitations of selectivity, specificity, and efficiency of existing artificial enzymes.
Researchers from the CSIR-Central Leather Research Institute (CLRI), working with the support of INSPIRE Faculty Fellowship and WISE Kiran Fellowship of the Department of Science and Technology (DST), investigated the chemistry at the interface of proteins and nanozymes to push the limits of artificial enzymes.
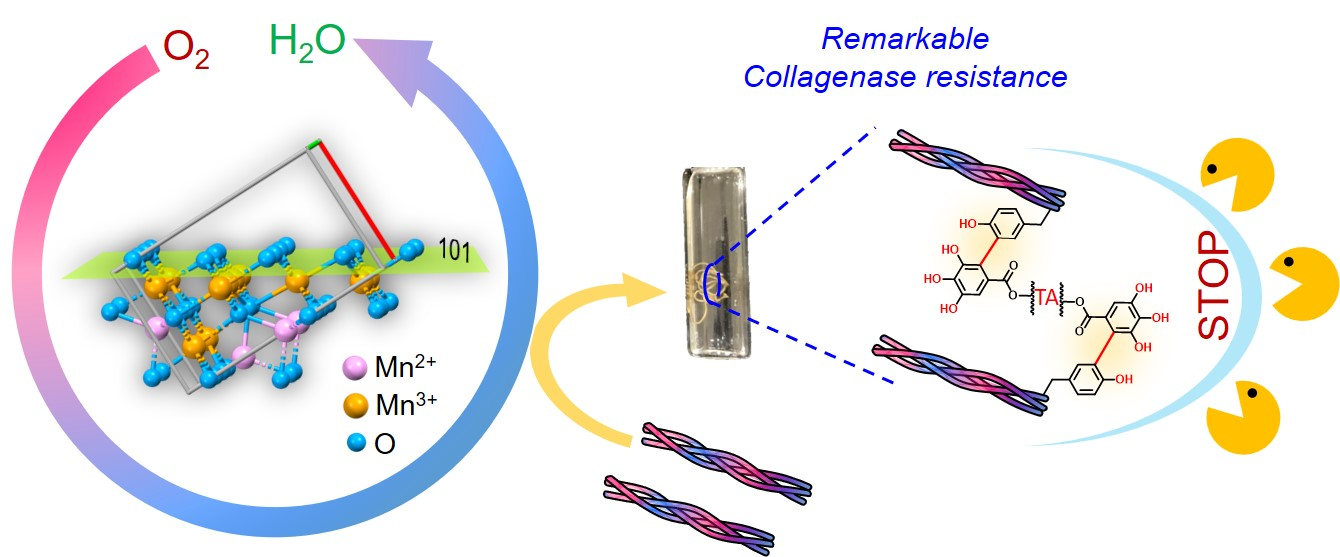
Dr. Amit Vernekar and his PhD students, Mr. Adarsh Fatrekar and Ms. Rasmi Morajkar have probed the crucial role played by manganese-based oxidase nanozyme (MnN) in stitching collagen, a vital structural protein in various biological tissues, through a covalent process known as “crosslinking” to produce biomaterials.
In a paper published in Chemical Science, journal of the Royal Society of Chemistry, they showed that MnN can activate collagen with the help of oxidase nanozyme and facilitate the covalent crosslinking of its tyrosine residues using only a trace amount of tannic acid under mild conditions, all the while maintaining the protein's triple-helical structure.
This approach not only showcases the novel prospects of nanozymes but also delivers an effective strategy to confer a remarkable 100% resistance to collagenase degradation, a significant challenge for the long-lasting use of collagen-based biomaterials.
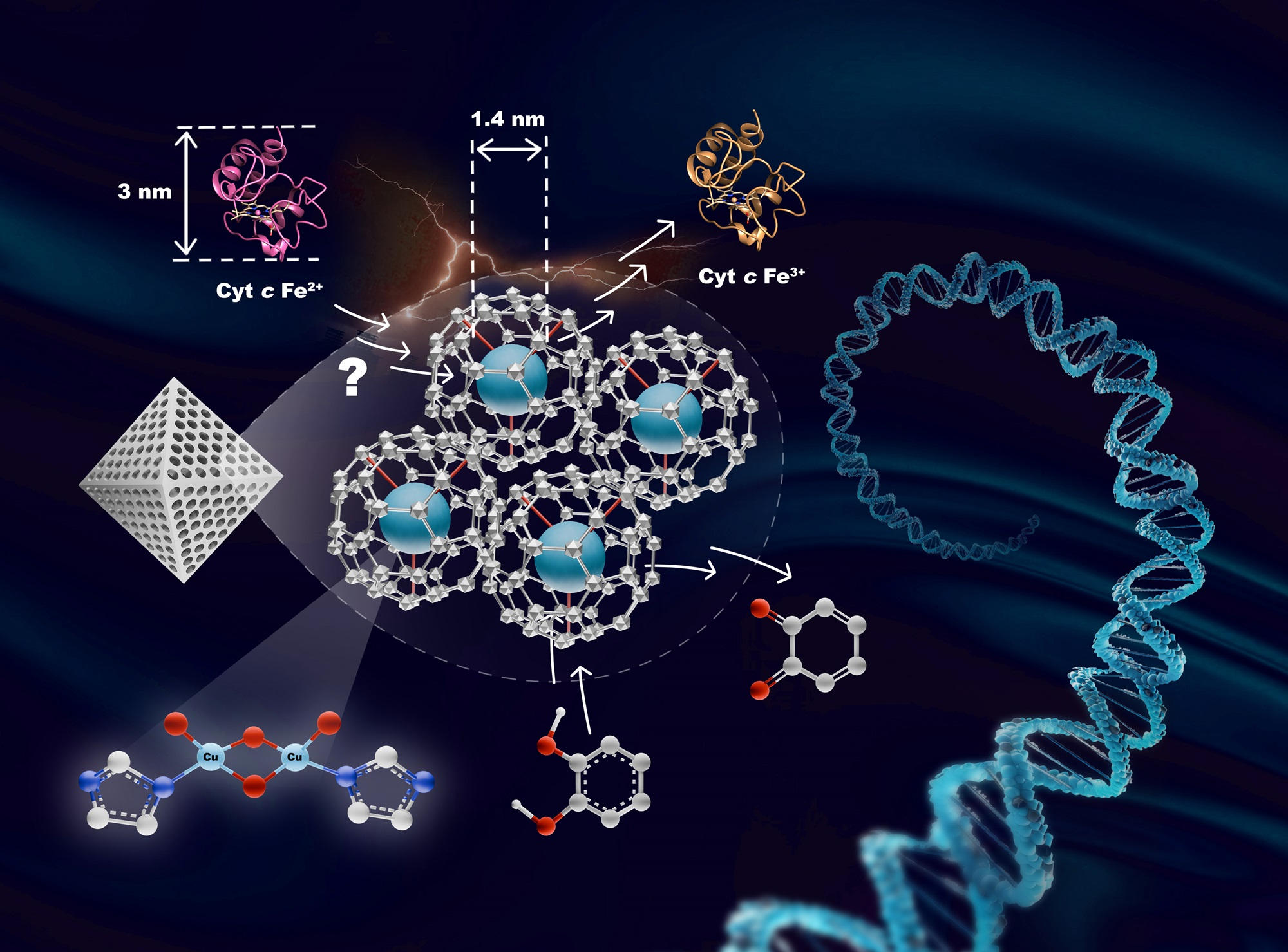
In another research, the scientists have designed a bis-(μ-oxo) di-copper active site installed within the pores of metal-organic framework (MOF-808) to serve as an analogy for enzyme binding pockets and address the persistent challenges of selectivity, specificity, and efficiency in nanozymes.
Their findings also published in Chemical Science illustrate that while this catalyst-by-design strategy effectively controls substrate dynamics and reactivity, it inadvertently compromises oxidase selectivity when small proteins, such as cytochrome c, which are larger than the pore opening of MOF-808, attempt to access the active site. This work exemplifies the need for careful consideration in the meticulous design of artificial enzymes related to nanomaterials, as the refined balance between desirable and undesirable reactivity in artificial enzymes is crucial for medicinal applications.
The research has expanded the repertoire of substrates to include complex biological molecules like collagen, pushing the limits of nanozymes beyond their known chemistry in functioning with small molecule substrates. This broadening of focus is important as it opens new avenues for the development of biomaterials for therapeutic applications, particularly those requiring intact structural properties. Through their research, they aim to establish guidelines for developing selective, specific, and highly active next-generation artificial enzymes for biomedical applications
The novelty of their work lies in its dual approach: first, by establishing a new paradigm for the interaction of nanozymes with structural proteins, and second, by highlighting the importance of substrate selectivity in the design of future artificial enzymes. These findings collectively contribute to a more refined understanding of nanozyme chemistry, essential for advancing their utility in biotechnological and therapeutic contexts.
The study redefines collagen biomaterial development with enhanced stability and durability.
Publication link: https://pubs.rsc.org/en/content/articlepdf/2024/sc/d4sc03767g
https://pubs.rsc.org/en/content/articlepdf/2024/sc/d4sc02136c