 Gelation process is ubiquitous in many industries as well as in day to day activities. Simple adhesives such as epoxy glue and even food materials such as Jello, agar gel containing products, bread dough, aqueous solutions of whey protein, carrageenan, and gellan gum and so on undergo a sol-gel transition process.
Gelation process is ubiquitous in many industries as well as in day to day activities. Simple adhesives such as epoxy glue and even food materials such as Jello, agar gel containing products, bread dough, aqueous solutions of whey protein, carrageenan, and gellan gum and so on undergo a sol-gel transition process.Dr. Khushboo Suman and Dr. Yogesh M. Joshi, scientists at IIT Kanpur in their study published in the Journal of Rheology, have monitored the sol-gel process in two gel-forming systems with distinctly different microstructure and gelation mechanism and experimentally proved for the first time that there is a methodical way by which these physical parameters evolve as such gel formation takes place.
Their study supported by the Science and Engineering Research Board (SERB), a statutory organisation of the Department of Science and Technology (DST), allows one to have complete information about sol-gel transition by carrying out very few tests. This will enable the scientific community to have apriori information about the characteristic properties of the material at each stage during the sol-gel transition without having the trouble of conducting multiple experiments at every stage, thereby saving resources.
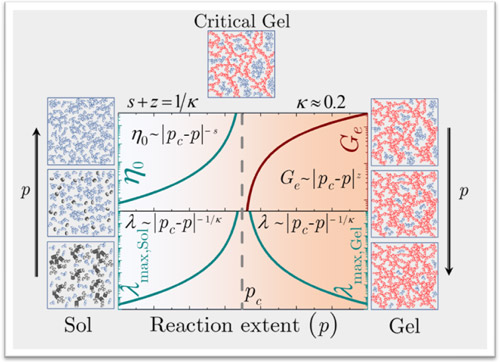
While undergoing sol-gel transition, the system passes through a unique state, the critical gel point, where the system forms the weakest 3-dimensional percolated network structure, which is fractal in nature.
One of the fascinating aspects of their study, on sol-gel transition, is experimental validation of the universal scaling behavior about the critical gel point, as proposed by the theoretical analysis of Stauffer and de Gennes (Physics Nobel Laureate 1991) back in the 1970s.
Consider a process in which small molecules (individual particles) connect with each other to form large cross-linked molecules (clusters). As the crosslinking (cluster formation) process continues, a state comes when the molecule spans the entire 3D space by forming a percolated network. The state at which the percolated network is weakest is known as a critical state. The network has a fractal structure at the critical state. As the process continues, the network becomes denser. The process may occur as a function of time or with a change in temperature, which is called as a gelation variable. As such percolation process progresses, various physical parameters, such as viscosity, modulus, relaxation time, and so on, undergo evolution. The network formation process is usually divided into two parts: before and after the critical gel state and is respectively termed as pre-critical gel state and post-critical gel state. The progress of network formation is measured in terms of the distance from the critical gel state in terms of the gelation variable independently in the pre and post-critical gel state.
The scientists have shown that irrespective of the nature of gel (molecular or particulate) or nature of crosslinking (chemical or physical), various physical properties depend on distance from the critical gel state in terms of gelation variable, including the universality in the interrelation between these dependencies. They observed that as the critical gel point is approached from the liquid side, the viscosity ( ) undergoes divergence.
On the other hand, beyond the critical point, as the system enters into the post-gel state, the equilibrium modulus ( ) of the system goes on increasing. Furthermore, they observe that the relaxation timescale ( ) diverges symmetrically on either side at the critical gel state. The two important results delivered through this work includes the universality in the application of the scaling relations to any gel-forming system, and the knowledge of any two exponents can describe the complete rheological response (response in terms of the physical parameters such as viscosity, modulus and relaxation time) of the material during the entire process of sol-gel transition. The universal scaling behavior established by the present work offers an simplified approach in understanding the complex process of sol-gel transition by a gel-forming material.
“The work by Suman and Joshi is interesting example of the first rate transnational science in that here a deep scientific analysis paves the way for understanding, determining, testing and controlling the complex process of gel formation in a variety of everyday products from cosmetics to food to paints to pharmaceuticals to adhesives,” said Prof Ashutosh Sharma, Secretary, DST.
Publication link:
https://doi.org/10.1122/1.5134115
https://doi.org/10.1122/1.5134115
For more details, please contact, Prof. Yogesh M Joshi, email: joshi[at]iitk[dot]ac[dot]in .






























