Scientists have fabricated monolayers of pure myelin basic protein (MBP), a major protein component of myelin sheath, which is a protective membrane that wraps around the axon of nerve cells and acts as a model protein in studying diseases like multiple sclerosis (MS).
MBP helps in compactification of the myelin sheath, and the fabricated tailored monolayers can give an in-depth understanding of the role of MBP in forming multi-lamellar myelin sheath structure as well as preserving the integrity, stability, and compactness of the sheath.
A research group from physical sciences division of the Institute of Advanced Study in Science and Technology, Guwahati, an autonomous institute of North-East India under the Department of Science and Technology, used a technique called the Langmuir-Blodgett (LB) technique to form monolayers of pure myelin basic protein at the air-water and air-solid interfaces.
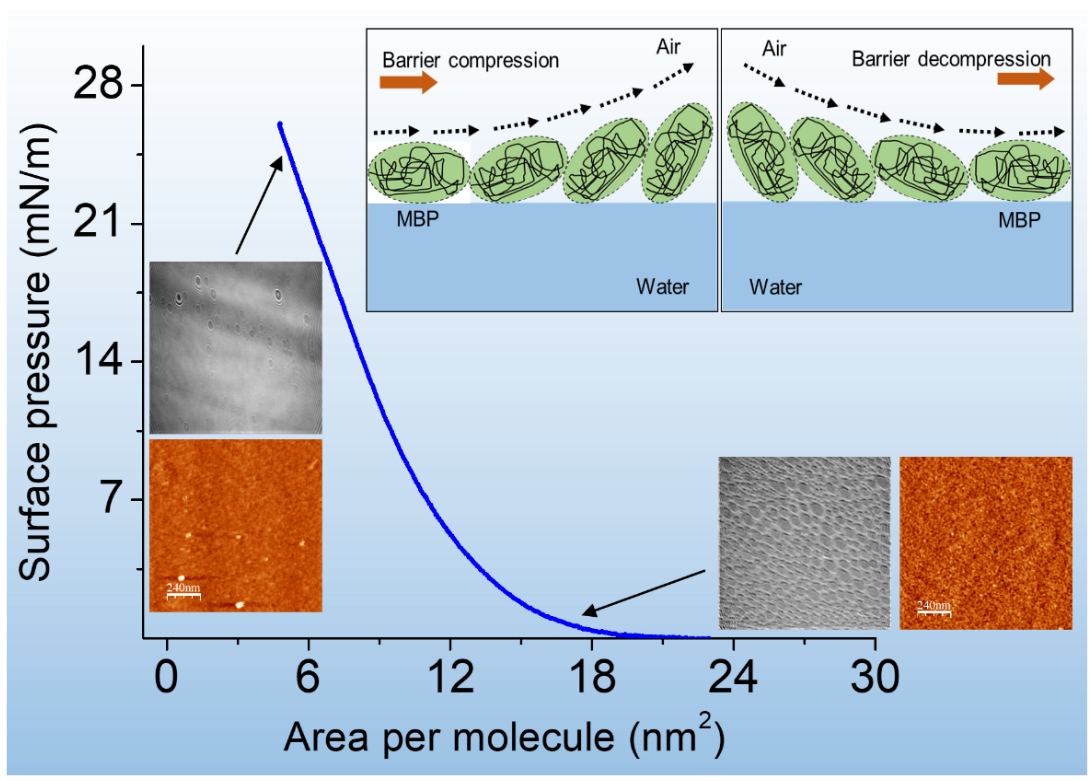
This research group is led by Dr. Sarathi Kundu, Associate Professor, along with Mr. Raktim J. Sarmah, a Senior Research Fellow, have explained the mechanism of formation of MBP while tracking the stability and rigidity of the protein films by tuning the subphase pH conditions. The reversible nature of the molecules confirms the flexibility of the films with respect to the pH conditions.
The behaviour of the protein under variable pH conditions were investigated from different areas of the monolayer formed at the air-water interface. The rigidity of the monolayers were correlated with the specific domains formed and the area occupied by the domains on the water surface.
The closely packed MBP layer formed at the air-water and also on solid surfaces fabricated by the LB method will be helpful in studying different chemical and physical properties in 2D in the vicinity of protein environment. The deposited LB films of MBP may also be considered as protein nanotemplates to crystallize proteins of interest. This research work was recently published in the journal of Colloids and Surfaces A: Physicochemical and Engineering Aspects under the reputed Elsevier publishers.
Publication link: https://doi.org/10.1016/j.colsurfa.2023.130973






























