Scientists have found a green and efficient chemical process for preparing amides directly from alcohol using a Covalent Organic Framework (COF) based photocatalyst that can revolutionize industrial manufacturing of pharmaceuticals and synthetic materials.
Amides are essential in chemistry, serving as key components in a wide range of organic compounds, including proteins, pharmaceuticals, and synthetic materials. Traditional amide synthesis methods often require high temperatures and harsh conditions, leading to significant environmental impact and inefficiency. These conventional approaches typically involve transition metal catalysts and generate substantial waste, prompting the need for more sustainable alternatives.
Researchers from S. N. Bose National Centre for Basic Sciences, an autonomous Institute of the Department of Science and Technology (DST), have introduced a novel method for synthesizing amides from alcohols using a Covalent Organic Framework (COF) as a photocatalyst under red light irradiation. This catalytic method can be helpful in chemical processes across various industries, including pharmaceutical manufacturing, materials science, and green chemistry - offering a more sustainable, efficient, and recyclable approach to creating vital chemical structures.
The advantages of this method include mild reaction conditions, high efficiency, excellent recyclability, and the practicality of red-light activation, which is less harmful and penetrates more effectively, making it suitable for large-scale applications. Additionally, the tolerance of COFs to various functional groups broadens their applicability to challenging substrates, such as secondary amides, which are difficult to synthesize using traditional catalysts.
The newly developed method uses the redox-active TTT-DHTD COF, which has been designed with high-density organic moieties, namely dithiophenedione, which is crucial for trapping photogenerated electrons (Scheme 1). This feature enables the COF to efficiently facilitate hydrogen atom abstraction reactions. The ability of the COF to absorb light across the visible spectrum, coupled with its narrow band gap, makes it particularly effective for generating excitons, which are essential for dehydrogenative coupling reactions. Upon red light absorption, the COF undergoes a photochemical reaction that generates excited states capable of initiating the dehydrogenation of alcohols, resulting in amide formation through coupling with amines. The process benefits from the stability and recyclability of COFs, making it a robust catalyst for repeated use.
The implications of this research are significant. In the pharmaceutical industry, this method could streamline drug production, reduce costs, and eliminate metal contamination. In materials science, it could enable the development of new polymers and materials with amide linkages, expanding the range of materials for various applications. Further research may optimize the COF structure for even better performance and stability, and scaling up the process for industrial applications will be crucial to realizing its full potential.
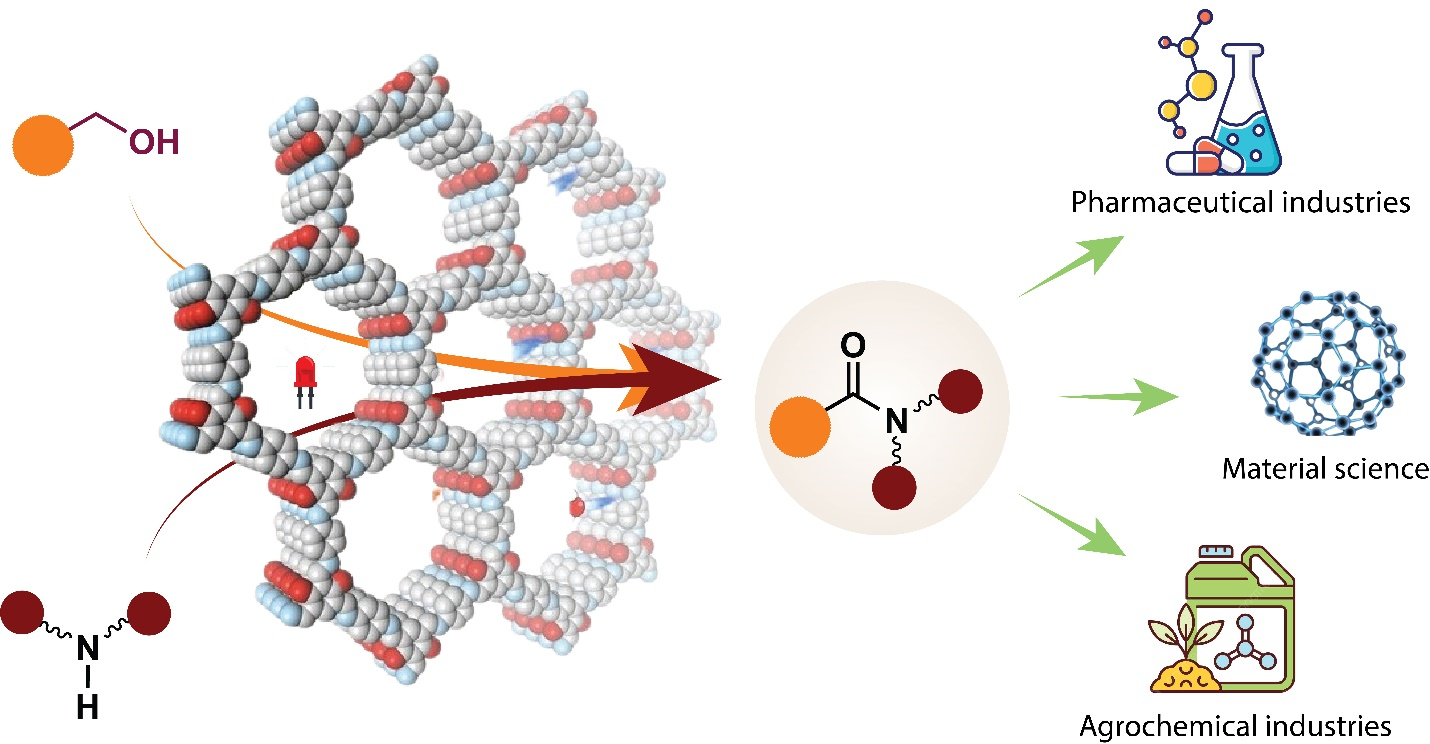
Scheme 1. Scheme of synthesis of amides using covalent organic frameworks as heterogeneous photocatalysts.
The development of the TTT-DHTD COF-catalysed method for sustainable and green amide synthesis marks a significant advancement in chemical catalysis. By combining mild reaction conditions, efficient light activation, and excellent recyclability, this approach addresses many limitations of traditional methods and paves the way for more sustainable and efficient chemical processes. As research progresses, the impact of this breakthrough could extend across multiple industries, driving progress toward greener and more effective chemical synthesis.
Publication link; https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202410300






























