An inorganic material called Hafnium oxide (HfO2), so far used in optical coatings, may soon find use as efficient memory devices in computers, sensors and sonars as an effective alternative to silicon dioxide (SiO2).
Thin films of Hafnium oxide (HfO2), one of the most common and chemically stable compounds of the rare metal Hafnium and an appropriate component of integrated circuits, exhibits spontaneous electric polarization even in the absence of external electric field or a Ferroelectric material. This polarization could be reversed by applying an electric field in the opposite direction. This chance discovery opened up new possibilities for HfO2 as memory devices. These can replace conventional ferromagnetic material as RAMs in computers and other devices that would consume less power along with faster switching speed than conventional materials.
However, before the HfO2 thin films with ferroelectric properties can be put to use, the problem of scalability and retention of memory have to be solved.
To put ferroelectric (FE) HfO2 to use, it became necessary to stabilize the polar orthorhombic phases of the oxide as the other phases are not expected to show ferroelectricity. Several dopants were explored by scientist of Bhabha Atomic Research Centre (BARC), in association with S N Bose National Centre for Basic Science (SNBNCBS) an autonomous institute of Department of Science and Technology for this purpose and Gadolinium (Gd) was predicted found to be one of the potential dopants for this purpose.
It was proposed that a doped and confined HfO2 thin film undergoes phase transition to a non-centrosymmetric orthorhombic structure—responsible for the ferroelectric behaviour. The dopant could stabilize the FE phase even in thicker HfO2 films. This discovery greatly increased the scope of the uses of HfO2 with ferroelectric properties. A direct experimental evidence for the presence of the polar orthorhombic phase in doped thin films of HfO2 was also reported.
Their study published in Physical Chemistry Chemical Physics explained the role of the Gd-dopant in stabilizing the O-phase in bulk HfO2.
Using Density Fluctuation Theory (DFT) calculations the scientists established that the segregation of the Gd-dopant in the grain boundary is a thermodynamically favorable process and the solute drag effect (atomic scale effect which inhibits the crystal growth process) plays an important role in nucleation of the O-phase in bulk HfO2. Doping of Gd was found to be thermodynamically highly favorable under an oxygen-rich environment as calculations showed negative defect formation energies.
The corroboration of theory with experimental results confirmed the microscopic role of the Gd-dopants in stabilizing the O-phase in bulk HfO2. The researchers postulated that o-nucleation takes place in the grain boundary region and there exists a critical crystallite size to trigger the process of O-nucleation. The relative population of the O-phase is greatly influenced by the Gd concentration and hence, the properties of such a device can be tuned by proper adjustment of the Gd-dopant.
The study opens up a possibility of augmenting the application of this ferroelectric device not only as thin films but also in bulk dimensions.
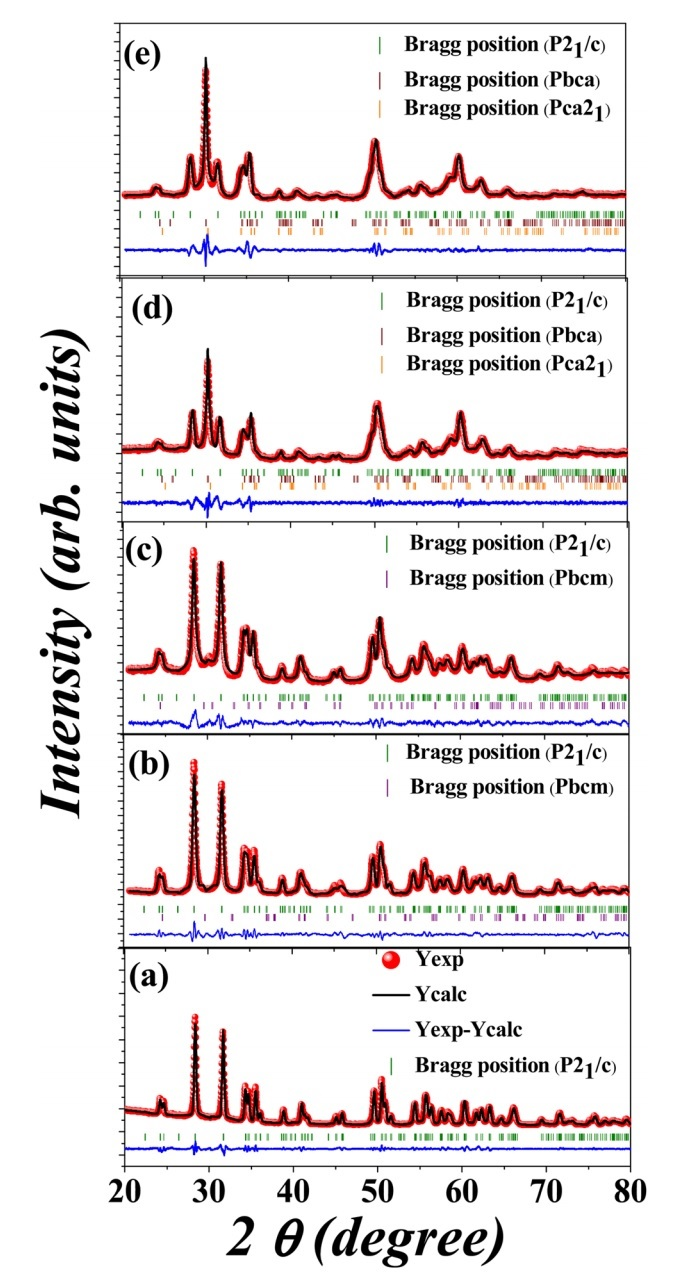
Fig 1. The Rietveld refinement of the experimental XRD data for (a) pure HfO2, (b) 1% Gd-doped HfO2,(c) 3% Gd-doped HfO2,(d) 4% Gd-doped HfO2, and (e) 5% Gd-doped HfO2 respectively.
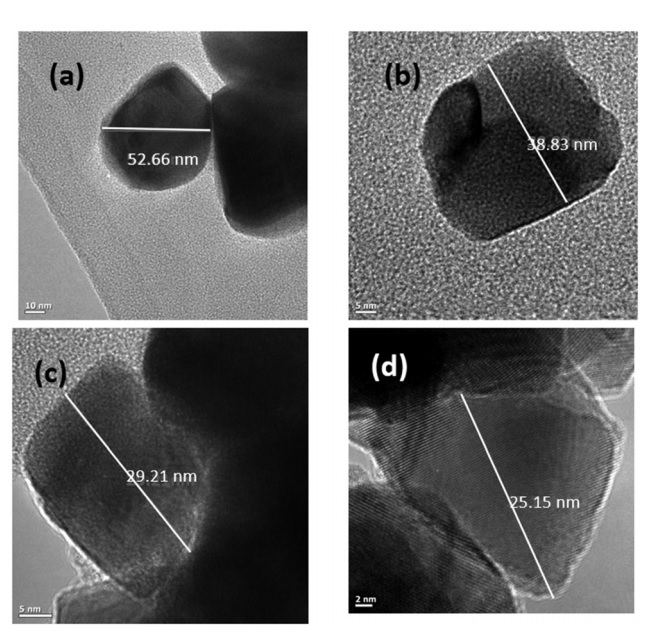
Fig 2. TEM images of pure and doped HfO2 samples. a) Pure HfO2; b) 1% doped; c) 3% doped; d) 5% doped






























